Muscle stem cells (MuSCs) reside in a complex niche around the terminally differentiated myofibres and are necessary for adult skeletal muscle growth and repair. Upon stimuli, they can both generate committed muscle precursors as well as self-renewing daughters that return to quiescence and replenish the stem cell niche. Signals from the niche microenvironment and the local milieu are well known to regulate stem cell kinetics of multiple tissues in vivo. During healthy aging, the wear and tear of skeletal muscle manifests itself by a degenerative loss of skeletal muscle mass and strength and is associated with detrimental effects to the aged niche that disrupts MuSC quiescence and reduces their self-renewing capacity and regenerative function. Thus, understanding the molecular mechanisms by which MuSCs undergo cell fate decisions, especially self-renewal, during muscle regeneration and aging is one of the fundamental goals of modern muscle research.
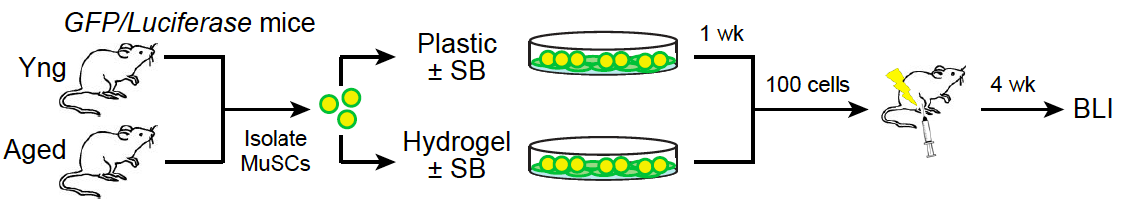
Fig 1. Rejuvenation of aged MuSCs. Ex vivo treatment of aged MuSCs with a p38 inhibitor (SB) in the context of a muscle- mimetic hydrogel restores their ability to regenerate muscle (engraftment) to be on a par with young MuSCs.
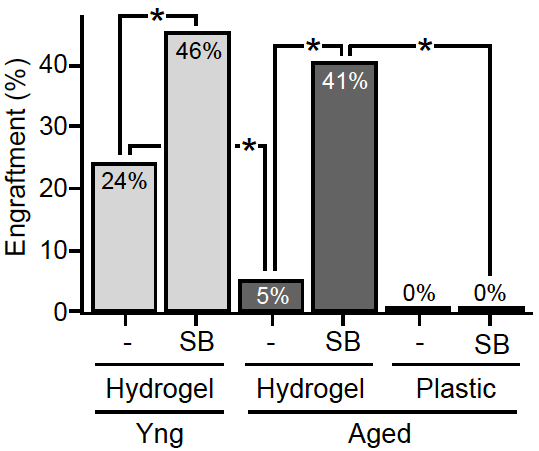
Our recent studies determined that in addition to the extrinsic alterations incurred by the aged MuSC microenvironment in the course of aging, MuSCs exhibit intrinsic cellular and molecular defects. The rarity and reduced proportion of functional MuSCs in aged muscle tissues highlights the need to increase the number of (expand) stem cells from this population that are capable of regeneration, in order to meet the goal of an autologous stem cell therapy. We show that the function of aged MuSCs can be pharmacologically restored by inhibiting the p38 mitogen-activated protein kinase (MAPK) signaling pathway. Notably, this treatment is efficacious specifically in the context of soft hydrogel cultures with biophysical properties similar to young muscle tissues underlying the synergistic effect of biochemical and biophysical niche cues.
Aged MuSCs treated ex vivo with the combined pharmacological/biomaterial strategy undergo extensive self-renewal leading to expansion of the functional MuSC population in culture. The resulting stem cell population has rejuvenated function and regenerates muscle on a par with young stem cells in vivo. This population confers long-term beneficial effects, evident from their ability to repopulate the stem cell reservoir and persist through serial transplantations. Most notably, transplantation of treated aged MuSC populations into aged recipient animals restores their strength to levels similar to young mice. Our findings indicate that an ex vivo rejuvenation strategy for autologous muscle stem cell therapy may serve to restore strength in cases of localized muscle damage in aged individuals.
In recent studies, we identified another biophysical MuSC niche cue that is altered in aged compared with young skeletal muscle tissue; niche architecture. In preliminary studies we identified a candidate molecular pathway responsible for maintaining normal niche positioning in young tissue that is incorrectly regulated in aged tissue. Hence, current work is focused on developing a strategy to restore aged MuSC function systemically by targeting niche architecture, a novel biophysical niche cue.