Regenerative Medicine Program and Spinal Cord Research Centre, Department of Physiology, University of Manitoba
Rationale: The involvement of free radicals and induction of oxidative stress in extensive cell death after brain and spinal cord injury is well documented. A variety of antioxidant molecules have been used to scavenge free radicals but have failed to produce significant improvement in clinical or pre- clinical trials, and therefore the need for novel therapeutic approaches remains a high priority. While reports of successful application of stem cells including those from our group for therapeutic purposes has raised the hope for finding a cure, the early waves of cell death is specifically unaffected by stem cell therapy. The resulting irreversible loss of neural cells and the endogenous stem cells contribute to permanent functional loss for the patients.
Approach: Our research is focused on therapeutic approaches to enhance tissue repair and endogenous regeneration. We use protein transduction technology to rapidly enhance the tissue concentration of Thioredoxin1 (Trx1). Increasing evidence indicate the importance of Trx1 in regulation of free radicals, inhibiting cell death and enhancing stem cells proliferation. We use a recombinant human Trx1 protein that is fused with the Tat peptide. This ensures a rapid and non- energy dependent drug delivery system for the damaged neural tissue after trauma.
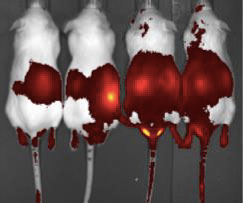
Tat-Trx treatment enhances Trx1 tissue distribution.
| Trx | Tat-Trx |
|---|---|
 |
|
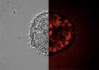
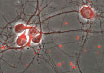
| Tat-Trx delivery results in intracellular delivery of Trx. (Tat-Trx is shown in red) | |
 |
|
 |
Methods: Clinically relevant rodent models of Brain and Spinal cord Injury as well as cultures of neuronal and neural stem cells are used in our studies.
Results and conclusion: Exciting results from our group indicate the therapeutic potential of Trx1 application for inhibition of cell death after neurotrauma. Trx1 also enhances neural stem cell proliferation in cultures as well as after direct delivery into the brain tissue. Our Tat-Trx1 delivery method results in a rapid and long-term accumulation of Trx1 in neural tissue and therefore represents a suitable drug delivery method. These results further support our hypothesis that enhancing tissue reducing capacity may have potential therapeutic application for the CNS repair after trauma.