1. Department of Speech Pathology and Audiology and Institute for Stuttering Treatment and Research, Faculty of Rehabilitation Medicine, University of Alberta, Edmonton, AB; 2. Departments of Health Sciences and Speech, Language and Hearing Sciences, Sargent College of Health and Rehabilitation Sciences, Boston University, Boston, MA; 3. The Research Institute at Nationwide Children’s Hospital, Columbus, OH
Introduction: Developmental stuttering is a communication disorder that starts in children aged 2-5 years old and is characterized by involuntary sp eech sound repetitions and prolongations as well as silent blockages of airflow that interrupt the fluent production of speech [1]. For the 350,000 Canadians who stutter, the inability to produce fluent speech results in social isolation, victimization by bullies, reduced academic performance and limited opportunities for advancement in the workforce [2-4]. Stuttering treatment is costly and, unfortunately, effective for only a subset of those with the disorder [5]. The cause is unknown and there is no cure.
My research project is a systematic investigation of the genetic contributions to developmental stuttering that leverages a large existing clinical population available through the Institute for Stuttering Treatment and Research (ISTAR) in the Faculty of Rehabilitation Medicine at the University of Alberta, of which I am the director. The current project is a foundational component of my overall research program that aims to understand the neurobiological origins of speech production, stuttering and associated disorders of speech motor control at multiple levels of organization. Recent advances in neuroimaging of children who stutter have helped to identify abnormalities in the neural networks governing speech production, which contribute to the impairment of speech fluency [6-9]. While such results have enhanced our understanding of the neurological correlates of the disorder it is clear that susceptibility to stuttering is highly heritable, and a comprehensive understanding necessitates complementary investigations at the behavioural, neurological and genetic levels [10-12]. My current research project is a crucial first step towards the identification of genetic variation associated with stuttering phenotypes and toward a future understanding of how these variations impact brain development and neural circuitry. The ultimate goal is to leverage this knowledge for improved clinical treatment via genetic risk assessment for dysfluent speech and the development of novel pharmaceutical and neurorehabilitative interventions.
Objectives: As shown in the workflow in Figure 1, the current project aims to accomplish three critical objectives:
Investigate family aggregation of developmental stuttering and its co-occurrence with other developmental and genetic disorders via collection of a detailed family health and developmental history and a standardized speech assessment. It is expected that completion of this objective will result in a novel contribution to the literature on stuttering.
Based on family history, strategically classify probands into three pedigree categories (i) rare single- gene, high penetrance de novo mutation cases, (ii) familial forms that segregate a single mutation across generations and (iii) the remaining idiopathic cases. Based on probable yield, the first two groups of interest will be a priority for next-generation DNA sequencing for identification of rare genetic variants. This objective is to provide the necessary information for future funding applications to CIHR to perform the sequencing analysis on these selected cases.
Establish a suitably large database of DNA collected from 150 stuttering probands and 150 control probands and selected biological relatives as a necessary requirement for future molecular genetic proposals. The database will establish the feasibility of our group to collect DNA from a large population of stuttering probands in a reasonable period of time. We will leverage the database for funding from CIHR to pursue investigation of the common genetic variants that contribute to the broader population of idiopathic stuttering cases. We will use emerging techniques for characterizing disorder etiology, including genome-wide association and imaging-genetics methodologies, that require very large populations (1000 stuttering probands, 1000 controls) for sufficient statistical power. Our database will form the beginning of a repository of DNA samples that will be a valuable asset for the field as a means of replication and resolution of conflicts in the literature. Possession of the repository will establish my laboratory as a world leader for the facilitation of an international consortium for large-scale genetic investigations into stuttering and speech motor disorders, an entity frequently championed in the literature, but which has not yet come to fruition [10,11].
Progress to date (September 2013): The current project was successfully funded in June 2013 by the Women and Children’s Health Research Institute at the University of Alberta. In just 3 months we have collected saliva DNA samples from 63 stuttering probands and selected immediate family members (150 DNA samples in total). Data collection is ongoing and analysis of family aggregation of the disorder is in progress. The DNA from priority pedigrees will be sequenced and results shared at conferences and in publications. The database will be used to obtain funding from CIHR to further sequence the DNA of the broader population of people who stutter and obtain magnetic resonance imaging data from this population for the purposes of determining genetic influence on brain development.
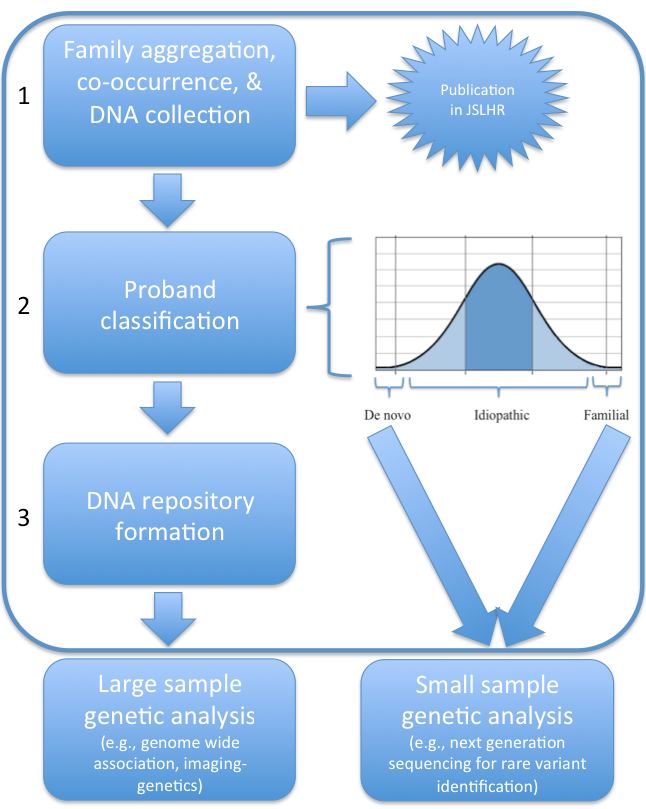
Figure 1. Figure 1. Project workflow. The current study will accomplish 3 important objectives (framed within the large box). First we are investigating the family aggregation of developmental stuttering and its co-occurrence with other disorders while simultaneously collecting DNA via saliva samples from participants. Completion of this objective will lead to a publication. Second, based on the family history collected for the family aggregation study, we will strategically classify probands into three groups based on probable yield for further genetic analysis funded by a federal science grant (CIHR, NIH etc). Third, all of the DNA collected will form the beginning of a large repository of stuttering DNA. The DNA repository will be used to secure funding for studies requiring large sample sizes such as a genome wide association study or imaging-genetics study to reveal associations with neurologically-based intermediate phenotypes.
References
- O. Bloodstein, N. Bernstein Ratner, A Handbook on Stuttering (Thomson Delmar Learning, Canada, ed. 6th, 2008).
- M. Langevin, N. G. Narasimha Prasad, A stuttering education and bullying awareness and prevention resource: A feasibility study. Language, Speech, and Hearing Services in Schools. 43, 344-358 (2012).
- G. W. Blood et al., Self-reported experience of bullying of students who stutter: Relations with life satisfaction, life orientation, and self-esteem. Percept. Mot. Skills. 113, 353-364 (2011).
- S. Davis, P. Howell, F. Cooke, Sociodynamic relationships between children who stutter and the non- stuttering classmates. J. Child Psychol. Psychiatry Allied Disciplines. 43, 939-947 (2002).
- M. Langevin, D. Kully, S. Teshima, P. Hagler, N. G. Narasimha Prasad, Five-year longitudinal treatment outcomes of the ISTAR Comprehensive Stuttering Program. Journal of Fluency Disorders. 35, 123-140 (2010).
- D. S. Beal et al., Speech-induced suppression of evoked auditory fields in children who stutter. Neuroimage. 54, 2994-3003 (2011).
- D. S. Beal, V. L. Gracco, J. Brettschneider, R. M. Kroll, L. F. De Nil, A voxel-based morphometry (VBM) analysis of regional grey and white matter volume abnormalities within the speech production network of children who stutter. Cortex. (in press).
- S. Chang, K. I. Erickson, N. G. Ambrose, M. A. Hasegawa-Johnson, C. L. Ludlow, Brain anatomy differences in childhood stuttering. Neuroimage. 39, 1333-1344 (2008).
- C. Weber-Fox, A. Hampton Wray, H. Arnold, Early childhood stuttering and electrophysiological indices of language processing. Journal of Fluency Disorders. (in press).
- S. J. Kraft, E. Yairi, Genetic bases of stuttering: the state of the art, 2011. Folia Phoniatrica Et Logopaedica : Official Organ of the International Association of Logopedics and Phoniatrics (IALP). 64, 34-47 (2012).
- S. Felsenfeld, Progress and needs in the genetics of stuttering. Journal of Fluency Disorders. 21, 77- 103 (1996).
- E. Yairi, N. G. Ambrose, Early childhood stuttering I: Persistency and recovery rates. Journal of Speech, Language, and Hearing Research. 42, 1097-1112 (1999).